M. Amin Arnaout Laboratory
Massachusetts General Hospital | Harvard Medical School
TOP NEWS
Mass. General study identifies path to safer drugs for heart disease, cancer—Massachusetts General Hospital News Articles, 23 March 2014
solving-the-problem-of-shape-shifters/—Harvard Gazette, 25 March 2014
Path to safer drugs for heart disease, cancer found by researchers—Science Daily, 23 March 2014
study identifies path to safer drugs for heart disease, cancer—Medical Breakthroughs, 25 March 2014
Cell signalling:The MIDAS touch—Nature Reviews Molecular Cell Biology, May, 2002
An Anthropomorphic Integrin—Science, October 12, 2001
More news...
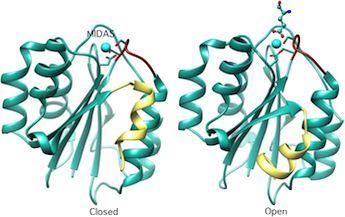
Crystal structure of the A-type domain from integrin CD11b in inactive (closed) and active(ligand-bound) conformations. MIDAS, metal-ion-dependent-adhesion site. Cell 1993;72:287; Cell, 80:631, 1995; Structure, 3:1333, 1995.
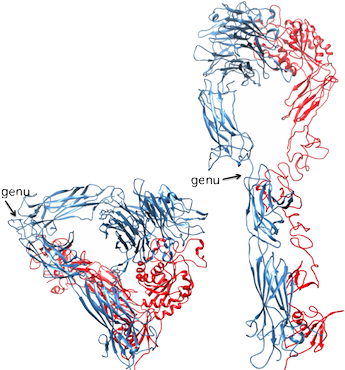
A ribbon drawing of the crystallized extracellular segment of αVβ3. The αV and β3 subunits appear in blue and red, respectively. The structure is severely bent at the kneelike "genu" (arrows). At right is a computer model of the straightened αVβ3, which was developed by extension (135 degrees) and rotation (120 degrees) of the bent structure at the genu. Science, 294:339, 2001.
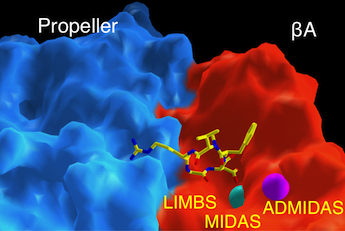
Surface representation of the ligand-binding site in integrin αVβ3 (αV subunit is in blue; β3-subunit is in red). The ligand is an Arg-Gly-Asp containing peptide, shown as ball-and stick model. Two metal ions at MIDAS and ADMIDAS are shown in cyan and magenta, respectively. Metal ion at LIMBS is buried. Science, 296:151, 2002.
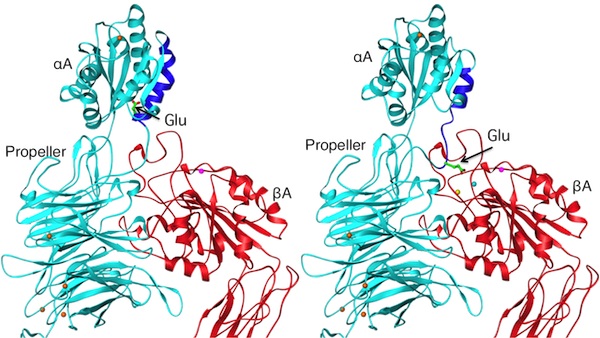
Ribbon diagram of a model showing an inactive (left) and an active (right) conformations of an αA-containing β2 integrin. A conserved glutamate at the base of the c-terminal α7 helix (blue) of the αA domain acts as an intrinsic ligand. Current Biology, 12:R340, 2002.
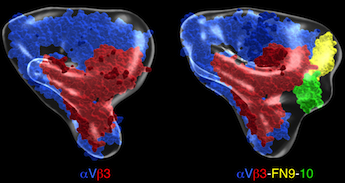
Pseudoatomic models of unliganded and fibronectin(FN)9-10-bound αVβ3. αV is in blue and β3 in red. Fn-9 and 10 are in yellow and green, respectively. J Cell Biol 168:1109, 2005.
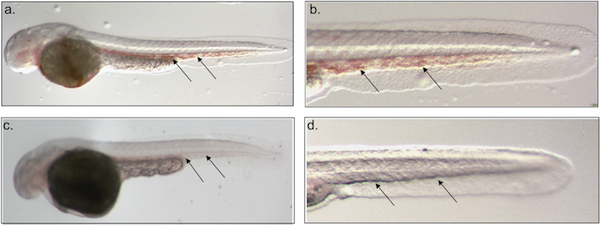
Loss of the transcription factor ZBP-89 results in a bloodless phenotype in zebrafish. (a-d) DAF staining of 48 hpf whole mount zebrafish embryos. Blood (arrows) is present in representative control (a, b) but not in ZBP-89 morphant (c, d). Embryos are shown at 4x (a, c) and 10x (b, d) magnification. Different embryos are shown in (c) and (d). Views are lateral with anterior to the left and dorsal to the top. Development, 133:364, 2006.
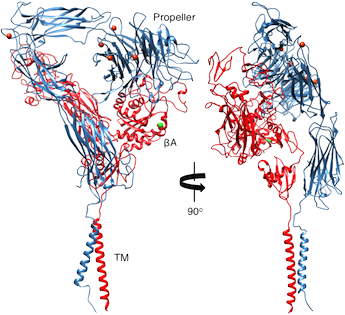
Ribbon diagram showing two views of a structure model of the complete αVβ3 ectodomain plus the TM domains. The orientation of the ectodomain relative to the TM domains, show that the ligand binding site is accessible to macromolecular ligands without unbending. αV and β3 are in blue and red, respectively. The metal ions at the α-genu and propeller are in orange. J Cell Biol, 186: 589, 2009.
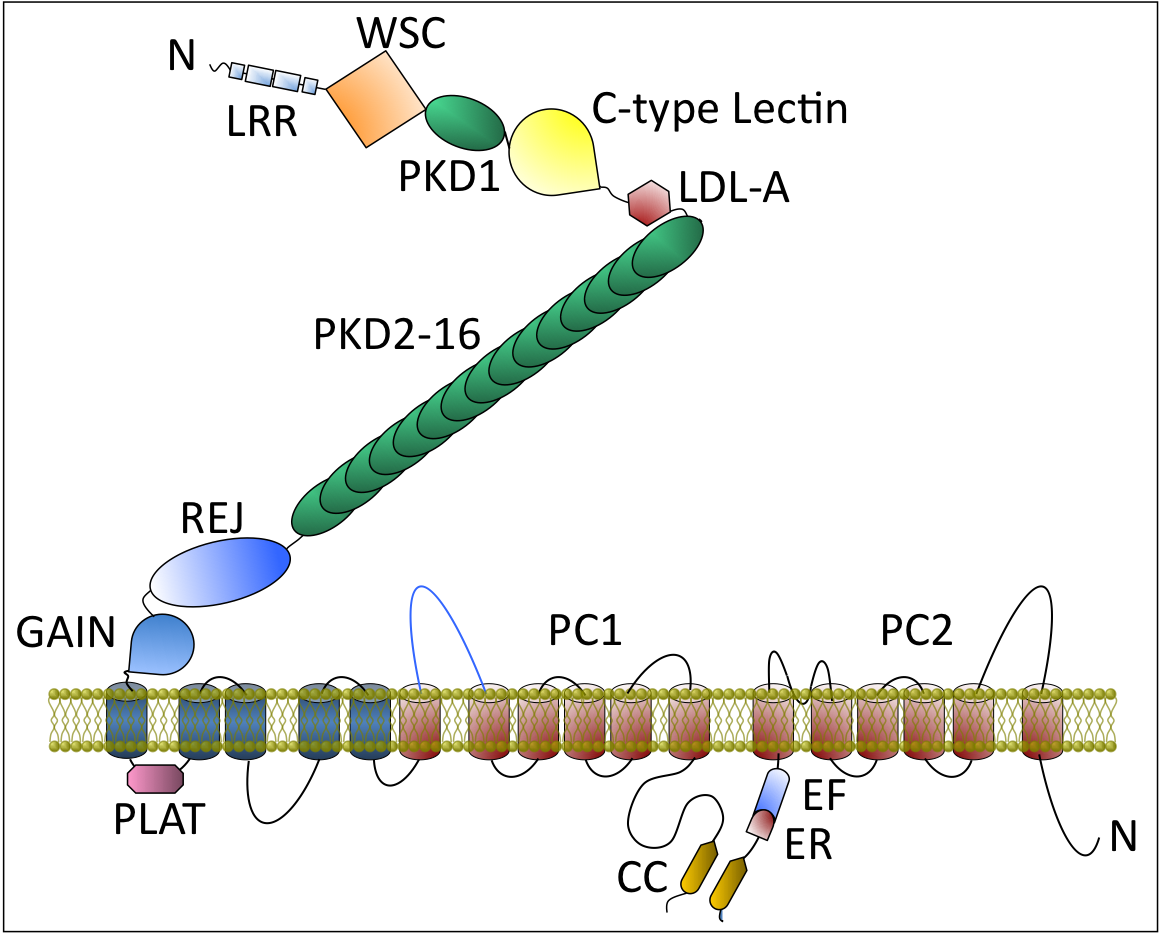
Schematic of PC1 and PC2, encoded by PKD1 and PKD2, respectively. PC1 is a multidomain glycoprotein with 11 putative transmembrane (TM) segments, the C-terminal six of which (colored in orange) bear homology to the six transmembrane segments of PC2. PC1 domains: LRR = leucine-rich region; WSC = cell wall and stress component; C-type lectin; Low-density lipoprotein receptor (LDL) A-like domain (LDL-A); PKD (Immunoglobulin-like domain); REJ = receptor for egg jelly domain; GAIN= G-protein-couple receptor Autoproteolysis INducing domain contains the GPS = G protein–coupled receptor proteolytic site (GPS) needed for PC1 activation; PLAT = PC1–lipoxygenase-α toxin domain. Inset: The PC1 schematic is drawn after atomic force microscopy imaging of PC1. Full-length PC1 appeared as two unequally sized blobs. The smaller one representing the N-terminal LRR, LDL-A, first PKD domain and C-type lectin and the larger one includes REJ, GAIN, all transmembrane domains, and cytoplasmic tail. The 35 nm string connecting the two blobs likely consists of the tandem PKD domains. PC2 domain: EF = calcium-binding motif consisting of two helixes, E and F. ER, endoplasmic retention signal.From, “Cystic Kidney Diseases”. In Cecil’s Textbook of Medicine. 25th edition, 2014. In press.
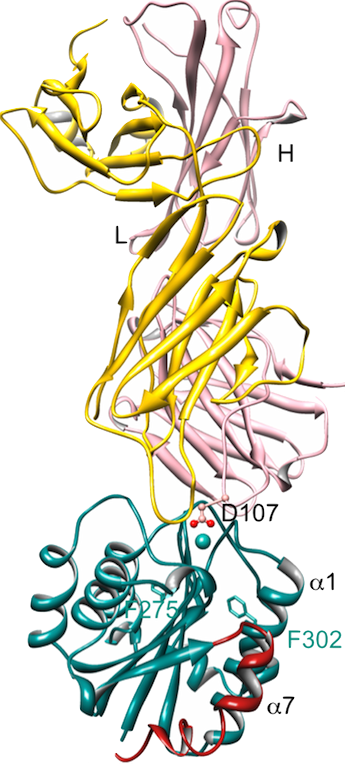
Ribbon representation of the structure of Fab107 in complex with high-affinity (Ile316/Gly) CD11bA domain. (A) Asp107 coordinates a MIDAS Ca2+ (cyan sphere) bidentately, converting high-affinity CD11bA into the low-affinity (closed) conformation. The observed unraveling of the lower segment of the α7 helix is caused by the helix-breaking Ile316/Gly activating mutation. J. Immunol. 187(12):6393,2011.
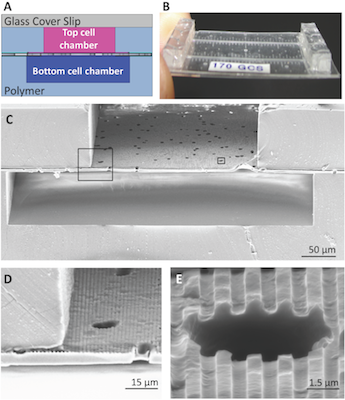
The topographically-patterned membrane assembled into a microfluidic device. The overall cross-sectional architecture of a device (A) was formed by a glass coverslip and two PDMS cell culture chambers separated by the patterned porous membrane. The thin nature of the device (B) allowed high-resolution imaging of cell populations on either side of the porous membrane. An SEM cross section (C) of the device shows the porous nature of the membrane separating the top and bottom chambers, while insets (D) and (E) show close-ups of well-defined groove topography coexisting with the porous architecture. Lab On a Chip 13:2311, 2013
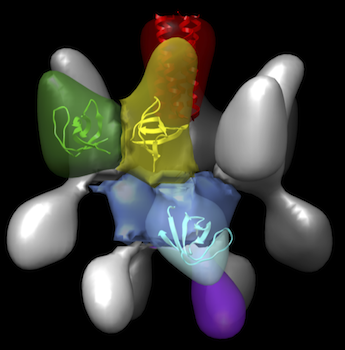
Surface-shaded, three-dimensional density map of CD2AP, a multidomain scaffolding protein that plays a critical role in maintenance of the filtration barrier of the kidney. CD2AP consists of a central coiled-coil domain, forming the tetramer interface, (colored red) surrounded by four symmetry related motifs each containing three globular domains, corresponding to the three tentatively assigned SH3 domains A-C (colored green, yellow and cyan, respectively in one subunit). The segment colored blue contains densities from each of the subunits. The segment colored purple likely contains C-terminal regions between SH3C and the coiled-coil domain, which include includes three poly-proline peptide regions as well as the CARMIL domain.J Am Soc Nephrol. Feb 7, 2014 [Epub ahead of print]
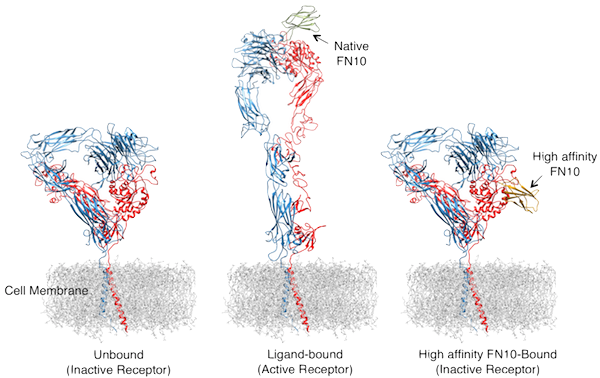
Structural basis for pure antagonism by an RGD-containing ligand. Illustration of the shape of an unliganded integrin (left), and the activating changes in its overall shape upon binding of native FN10 (or a ligand mimicking molecule) (center), causing cells to become sticky. In contrast, binding of high affinity FN10 did not produce these changes, thus acting as a pure antagonist of the integrin (right). The two peptide chains αV and β3 that make up a single integrin receptor αVβ3 are colored blue and red, respectively. Nature Struc Mol Biol March 23, 2014 [Epub ahead of print]; doi:10.1038/nsmb.2797.
MORE NEWS
Path to safer drugs for heart disease, cancer found by researchers—ScienceDaily, 23 March 2014
RECEPTOR–LIGAND INTERACTIONS—the signaling gateway, 2002
Mass. General study identifies path to safer drugs for heart disease, cancer—Eurekalert, 23 March 2014
Scientists from the Division of Nephrology have deciphered the detailed structure of integrin αVβ3—, Massachusetts General Hospital News Articles, March 7, 2002
Investigators from Division of Nephrology Report a Major Scientific Breakthough!—, Massachusetts General Hospital News Articles, September 6, 2001
Cell Adhesion Receptor Caught on Bended Knee—Harvard FOCUS, September 14, 2001
Role Found for Protein in Kidney Disease—Harvard FOCUS, January 12, 2001
Integrin binding revealed—Nature Structural Biology, 2: 181,1998
Infection Fighter Discovered—New York Times, March 26, 1993
 Dr. M. Amin Arnaout, MD
Dr. M. Amin Arnaout, MD
Professor of Medicine, Harvard Medical School
Director, Leukocyte Biology & Inflammation Program
Director, Structural Biology Program
Department of Medicine
Massachusetts General Hospital
Principal Investigator, Harvard Stem Cell Institute
Our laboratory is interested in elucidating the molecular basis of human disease and in using this information to guide development of new and safer therapies. We utilize state of the art technologies, including genetics, genomics, biochemistry, cell biology, structural and computational biology and animal models of disease.
PROJECTS
Cell-matrix Interactions: Structure-activity Relationships in Integrins
Integrins are αβ heterodimeric cell adhesion receptors of metazoa consisting of a bilobular head and two legs that each spans the plasma membrane once. Integrins are unusual receptors, as they normally exist on the cell surface in an inactive state, unable to engage physiologic ligand. This is critical for integrin biology, for example, it allows patrolling blood platelets and immune cells to circulate with minimal aggregation or interaction with the vessel walls. Physiologic stimuli (e.g. chemokines), acting through the short integrin cytoplasmic tails, induce allosteric changes in the large extracellular domain required for extracellular ligand binding (“inside-out” activation). Binding of physiologic ligands then induces “outside-in” signaling by initiating additional shape changes in the extracellular domain, triggering changes in how sticky cells become. Disruption of these processes can make cells more sticky, leading to clogged arteries, pathological inflammation, autoimmunity, fibrosis or cancer, or slippery, leading to bleeding or infections. Our studies aim at elucidating the structural changes in integrins at the atomic level and using this information in a structure-based approach to design new and safer drugs to treat a wide variety of diseases.
Autosomal Dominant Polycystic Kidney Disease (ADPKD)
ADPKD is the most common monogenic disease in humans, caused by dysregulation in diameter of tubular structures such as kidney tubules and blood vessels. ADPKD is caused by defects in one of two genes, PKD1 or PKD2. ADPKD leads to progressive loss of kidney function, and can result in catastrophic brain hemorrhage from ruptured vascular cysts. We have shown that defective PKD1 causes cysts in a mouse model, and that it cooperates with PKD2 to mediate calcium influx into cells, thus regulating cell migration, polarity and proliferation. Ongoing work is aimed at elucidating factors that regulate tube diameter during organ development, using zebrafish as a convenient animal model.
Cell Fate Determination in the Hematopoietic System
The hematopoietic system originates from a small population of self-renewing hematopoietic stem cells that differentiate into the various erythroid, myeloid, and B and T lymphoid lineages. The hematopoietic lineages tend to be specified in a stepwise process of binary decisions, dependent on particular genetic programs under control of transcription factors. The lineage-specifying and autoregulatory factors PU.1 and GATA1 form a master genetic switch that is responsible for determining the myeloid/lymphoid and erythroid lineages, respectively, commonly acting in concert with lineage-restricted factors such as SCL/TAL1 and CCAAT/enhancer binding protein α (C/EBPα). We have shown that ZBP-89 (Zfp148), which belongs to a novel class of GC-rich binding transcription factors, regulates developmental fate of hemangioblasts, the precursors of hematopoietic and vascular stem cells at the embryonic stage. ZBP-89 also regulates stress hematopoiesis in the adult bone marrow, revealed in experiments using conditional ZBP-89 knockout mice. Ongoing studies are aimed at elucidating the underlying mechanisms.
Kidney Regeneration
Despite decades of research and advances in patient-care, mortality rates for patients with acute kidney injury have not significantly decreased. The mammalian kidney possesses the inherent potential for regeneration and recovery of tubular function following acute injury, through recruitment and proliferation of surviving tubular epithelium. However, limitation in the number of surviving tubular cells commonly leads to progressive loss of renal function. The signaling pathways regulating tubular epithelial cell response to ischemic or toxic injury are incompletely understood. Stem cell antigen-1 (Sca-1, also called Ly6a) is a glycerophosphatidylinositol (GPI)-anchored protein, commonly used as a marker for the identification and isolation of stem cell populations. We have shown that Sca-1 has a wider cell distribution in the adult kidney. Ongoing efforts are aimed at identifying the role Sca-1 plays in the adult kidney, using in vitro and in vivo models of tubular cell injury.
Engineering Kidney-assist Devices
Accumulation of excess fluid and toxic metabolites are invariant consequences of kidney failure. Over the long-term, kidney problems have significant repercussions on cardiovascular system as well as on other organ systems. We are working with our engineering colleagues towards development of microfluidic devices and methods of filtering a liquid solution, which may find industrial or medical applications. For example, such microfluidic devices provide particular advantages in blood dialysis and may be developed into portable kidney assist devices.
MEMBERS
Principal Investigator
M. Amin Arnaout, MD
Faculty
Brian D. Adair, PhD
José Luis Alonso, PhD
Xiangen Li, MD, PhD
Jian-Ping Xiong, PhD
Jiaojiao Zhang, PhD
Current Postdoctoral Fellows
Johannes F. Van Agthoven, PhD
Troy Camarata, PhD
Wassim ElJouni, PhD
Bhuvaneshwari Mahalingam, PhD
Mujib Rhaman, PhD
Xianliang Rui, PhD
Xingzhi Li, MD
Students
Mahmoud Alhaj, MS
Laboratory Manager
Zhiping Ding, PhD
POSITIONS
If you are interested in applying for a postdoctoral fellowship position, or if you are a Harvard PhD student interested in a laboratory rotation, please e-mail your CV (for students and postdoctoral fellows) and reference letters (for postdoctoral fellows) to: aarnaout1@mgh.harvard.edu
CONTACT
Post
M. Amin Arnaout Laboratory
CNY 149 Suite 8208
Massachusetts General Hospital
149 13th Street
Charlestown, MA 02129